Cipla Share Price Drop 8% as USFDA Warning Letter Dampens Sentiment in Pharma Sector

Cipla Share Price
Cipla’s Market Turmoil: Unpacking the 8% Plunge Amidst USFDA Warning
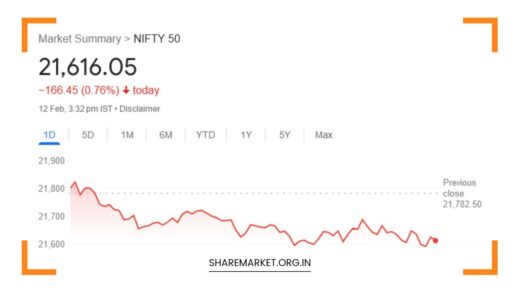
The morning of November 23 held promise for pharmaceutical giant Cipla as its stock opened with gains at Rs 1272.95 on the Bombay Stock Exchange (BSE). However, this optimism was swiftly overshadowed by a stark descent, with shares plunging by 8 percent during the day.
The catalyst for this market turbulence was a stern warning issued by the United States Food and Drug Administration (USFDA) directed at Cipla’s Madhya Pradesh unit.
This article delves into the intricate details of the events that transpired, exploring the factors contributing to the market reaction, Cipla’s response, and the broader implications for the pharmaceutical industry.
The Morning Momentum
Cipla’s trading session on November 23 began on a positive note, with an opening price of Rs 1272.95 on the BSE.
This initial uptick suggested a favorable start for the day, fostering hopes among investors. However, the momentum could not be sustained for long, as the day unfolded with unexpected challenges.
A Swift Descent: The 8% Plunge
Despite the morning optimism, Cipla found itself facing a rapid decline in its shares, ultimately reaching a low of Rs 1178.35, marking an 8 percent decrease.
Both the National Stock Exchange (NSE) and BSE mirrored this descent, with the stock opening at Rs 1270 and falling to a low of Rs 1178.10 on the NSE. The 52-week high and low for Cipla shares underscored the volatility, standing at Rs 1283 and Rs 852, respectively, on both BSE and NSE.
The Regulatory Trigger: USFDA Warning Letter
The root cause of Cipla’s market turbulence was a warning letter issued by the USFDA, specifically targeting the company’s Madhya Pradesh unit.
This cautionary letter followed a routine Good Manufacturing Practices (GMP) inspection at Cipla’s Pitampur unit. The regulatory scrutiny, while routine, had profound implications, contributing to the decline in investor confidence.
Insight into the USFDA Inspection
The USFDA inspection, conducted from February 6 to 17, 2023, aimed to assess Cipla’s adherence to Good Manufacturing Practices (GMP) at its Pitampur unit.
The warning letter highlighted several concerns, including data integrity issues, product complaints, and instances of microbial contamination.
These issues, when brought to light, triggered the market response witnessed on November 23.
Market Response and Dynamics
The market’s response to the USFDA warning was swift and unforgiving. Despite the initial gains, investors quickly recalibrated their expectations, leading to a significant decline in Cipla’s shares.
The warning, rooted in concerns about data integrity, product complaints, and microbial contamination, prompted a reevaluation of the company’s standing and future prospects.
Cipla’s Transparent Response
In response to the USFDA warning, Cipla adopted a transparent stance, promptly communicating the details to the stock markets.
The company acknowledged the issues raised during the inspection and assured stakeholders that it was diligently working to address the concerns outlined in the warning letter.
This proactive response aimed to reassure investors about Cipla’s commitment to rectifying the identified issues and maintaining compliance with regulatory standards.
Financial Resilience Amidst Regulatory Challenges
Despite the market turmoil, Cipla’s financial performance remained robust in certain aspects. The consolidated net profit for the September 2023 quarter surged by 43.3 percent year-on-year, reaching Rs 1,131 crore.
This financial performance indicated the underlying strength of Cipla’s operations and its ability to generate profits despite the regulatory challenges.
Milestone Achievement: Highest-ever Revenue from the American Market
The September quarter marked a milestone for Cipla as it achieved the highest-ever revenue of $229 million from the American market.
This achievement showcased the company’s global reach and its position as a significant player in the pharmaceutical industry.
However, the dichotomy of financial success against the backdrop of regulatory hurdles created a complex narrative for investors to navigate.
Analysts’ Perspective: ‘Buy’ Rating Amidst Caution
In the aftermath of these developments, analysts at Prabhudas Lilladher maintained a ‘buy’ rating for Cipla shares, with a target price of Rs 1,350 per share.
Their optimistic outlook was grounded in the expectation of a 17 percent Earnings Per Share (EPS) Compound Annual Growth Rate (CAGR) in the fiscal years 2023 to 2026.
This positive forecast provided a counterbalance to the immediate market pessimism, offering investors a strategic perspective amid the turbulence.
Identified Risks: FDA Escalation and Product Shortages
While expressing confidence in Cipla’s future prospects, analysts also acknowledged potential risks associated with the company’s current situation.
The looming possibility of further FDA escalations, particularly concerning the Indore unit, was identified as a major risk factor.
Regulatory uncertainties can create an environment of unpredictability, impacting investor confidence and the overall market perception of the company.
Additionally, the analysts highlighted the risk posed by potential shortages of key products in the US market. The pharmaceutical industry is inherently susceptible to supply chain disruptions, and any constraints in the availability of crucial products can have cascading effects on financial performance.
This risk factor underscored the delicate balance that pharmaceutical companies must strike in navigating regulatory landscapes and meeting market demands.
Navigating Regulatory Challenges: Complexity of the Pharmaceutical Industry
The intricate web of factors influencing Cipla’s market dynamics extended beyond immediate stock fluctuations. It delved into the company’s response to the USFDA warning and the measures taken to address the identified issues.
The warning, issued on the grounds of data integrity issues, product complaints, and microbial contamination, prompted Cipla to provide detailed information to the stock markets.
The Regulatory Landscape: Importance of Compliance
The USFDA inspection, while routine, emphasized the critical role of regulatory compliance in the pharmaceutical industry. Adherence to Good Manufacturing Practices (GMP) is fundamental for ensuring the quality, safety, and efficacy of pharmaceutical products.
Regulatory scrutiny, as evidenced in Cipla’s case, serves as a vital mechanism for maintaining industry standards and safeguarding public health.
Data Integrity Concerns: Pillars of Pharmaceutical Quality
The warning letter specifically pointing out data integrity issues at Cipla highlighted a fundamental aspect of pharmaceutical manufacturing.
Data integrity is crucial for ensuring the accuracy, consistency, and reliability of data throughout the product lifecycle. Any lapses in data integrity raise concerns about the quality and safety of pharmaceutical products, necessitating swift corrective actions.
Product Complaints: Addressing Quality Concerns
In addition to data integrity, the warning letter highlighted product complaints at Cipla’s Madhya Pradesh unit.
Product complaints encompass a range of issues, from defects in manufacturing to concerns raised by end-users about the efficacy or safety of a product.
Effectively addressing these complaints is essential for maintaining product quality and upholding customer trust.
Microbial Contamination: Risks to Sterility
Microbial contamination, another serious issue flagged by the USFDA, poses significant risks to patient safety. Contamination in pharmaceutical manufacturing can compromise the sterility of products, underscoring the importance of stringent quality control measures.
The emphasis on microbial contamination in the warning letter highlighted the critical need for robust manufacturing processes.
Balancing Act: Transparent Communication and Financial Success
Cipla’s response to the USFDA warning demonstrated a delicate balancing act. On one hand, the company adopted a transparent communication strategy, promptly informing stakeholders about the issues identified during the inspection and outlining its corrective measures.
On the other hand, the company reported robust financial performance, indicating resilience amidst regulatory challenges.
Analysts’ Forecast: Earnings Growth Amidst Uncertainties
The analysts’ optimistic outlook for Cipla’s future, with a projected 17 percent EPS CAGR in the fiscal years 2023 to 2026, provided a strategic lens for investors.
However, the cautionary notes about potential FDA escalations and product shortages underscored the uncertainties inherent in the pharmaceutical industry.
Conclusion: Navigating Complexity in a Dynamic Industry
In conclusion, Cipla’s experience on November 23, marked by an 8 percent decline in shares triggered by a USFDA warning, encapsulated the inherent volatility of the pharmaceutical industry.
The warning, rooted in concerns about data integrity, product complaints, and microbial contamination, prompted a swift market response that mirrored both apprehension and resilience.
While the market turbulence reflected immediate concerns, Cipla’s transparent response and robust financial performance offered a nuanced perspective. The analysts’ optimistic outlook, tempered by cautious considerations of potential risks, added layers of complexity to the narrative.
The pharmaceutical industry, by its nature, operates at the intersection of innovation, regulation, and market dynamics. Navigating this intricate landscape requires companies to balance short-term challenges with long-term strategies, ensuring not only compliance with regulatory standards but also sustainable growth in a dynamic market environment.
As Cipla addresses the issues outlined in the USFDA warning, the coming months will likely witness a convergence of regulatory developments, market reactions, and the company’s strategic initiatives.
Investors, analysts, and industry observers will closely monitor these dynamics, seeking insights into the resilience and adaptability of one of India’s leading pharmaceutical players in the face of regulatory headwinds.